How to remove industrial adhesive
Removing a cured adhesive can be tricky and the type will determine the method. The main methods are heat, chemicals and mechanical force.
Removing a cured adhesive can be tricky and the type will determine the method. The main methods are heat, chemicals and mechanical force.
We don’t always tend to think about how to remove an adhesive when we choose which one is the most suitable.
Usually, an adhesive is picked for its strength and adhesion. At times, however, it is necessary to dismantle a bonded detail. Perhaps for maintenance or because something has gone wrong. It can therefore be a clever idea to consider possible removal when choosing the adhesive.
Some products are developed to make them easily removed. An example is anaerobic threadlockers and flexible form-in-place gaskets, that have been formulated for low strength to be able to be removed without damaging softer metals.
Other adhesive types, such as structural adhesives, are formulated for maximum strength, temperature and chemical resistance. It goes without saying, removing them can be difficult, especially without damaging the substrates.
If possible, always try to remove an adhesive before it is fully cured.
If the adhesive has yet to cure, it is usually simple to remove. Wiping with a solvent such as iso-propanol can do the trick.
For a moisture curing silicone, SMP polymer or polyurethane, removing uncured material can easily become quite messy. In some cases, it can be better to wait until it has cured slightly and is dry (before the adhesion strength has built up) and then remove it mechanically without the messiness.
Removing adhesive that has already cured is a whole other story.
Curing adhesives are thermosets. These cannot be melted or dissolved. The only way to deform or break the ahesive (plastic) is permanently breaking the polymer chains it is made up from.
A reversible adhesive with high strength that will release under certain conditions has unfortunately not been invented yet (at least not in a commercial scale). Until then, we will have to settle for the methods and combinations of methods that exist:
The idea is to target what the adhesive is mot sensitive to and weaken it enough for disassembly. Let’s look at the best methods for different types of adhesives.
The formulations of anaerobics can be developed for dismantling by giving them a low strength so they can easily be dismantled. A common example is for threadlocking and threadsealing where the adhesive really only needs to withstand some vibrations but then can be dismantled with a simple wrench. Anaerobic gasket products are soft and are usually also quite easy to take apart using a screwdriver that is inserted between the pieces.
Other anaerobic adhesives can be stronger and meant to give a permanent bond. In these cases, the help of heat or chemicals can be necessary (make sure that your parts are not damaged by these!). By putting your pieces in an oven or using a blowtorch, the strength of the adhesive is reduced. While it is still warm, the bond can be mechanically dismantled, but the strength will return again once it cools down. For safety, use a pair of heat resistant gloves.
If you still don’t manage to remove the adhesive, soak the while detail in solvent overnight before attempting again. Make sure that all solvent has evaporated before you heat it up.
When you have managed to dismantle the pieces, you may still have residues. For best results upon bonding the pieces back together, these should be removed as well. Try using a metal brush to rub it off and use a solvent such as acetone or methylene chloride if needed.
Another factor to consider is that even an anaerobic with low strength can give you a high bond strength if you are using it on large areas.
Cyanoacrylates (instant adhesive) can be difficult to remove as it is often used for rubber and plastic materials that are quite sensitive to both heat and chemicals themselves.
Generally, cyanoacrylates are quite brittle, and you can start by trying to mechanically break apart the pieces by method of peeling.
If possible, most cyanoacrylate bonds weaken considerably over 80 ˟C. Solvents such as acetone or soaking in warm water can also help.
Did you accidentally glue your fingers together?
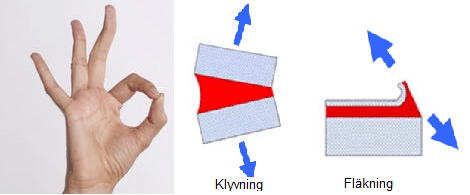
Use a peeling/cleaving motion to pry cyanoacrylate glued fingers apart.
Structural adhesives are developed for as high strength as possible and can therefore be tricky to remove. In addition, many of these types of products have high shear, tensile, peel and tear strength which makes breaking a bond difficult regardless of method.
The first thing to do is check the thermal resistance of the adhesive. It is usually stated in the Technical Data Sheet (TDS). From there, evaluate whether or not it would be possible to increase the temperature to these levels and break the pieces apart without damaging your substrates.
Solvents such as ethylene chloride can slowly de-bond an adhesive and can be an option for small bond areas. Using this method for larger areas would take a long time and the solvent would probably only help right at the edges.
If your substrates can withstand it, use heat (as high as possible, preferably over 200 C) to permanently degrade the adhesive. In other words, burn it off. This can be a plausible method if for example glass is bonded to glass. If glass is bonded to another material, such as a metal, it can be more difficult as differences in thermal expansion would probably result in the glass cracking.
Just as for structural adhesives, the alternative is soaking in solvent. This method is not to recommend for plastics such as polycarbonate or Plexi glass since solvent will also damage the plastic.
In some cases, UV curing adhesives can absorb a lot of water. Check for data on water absorption in the Techical Data Sheet of your product. If it is high, the adhesive can soften in a water bath so that it can be scraped off. Note that the strength will return as the adhesive goes back to normal again.
It is usually quite easy to mechanically take apart pieces bonded with silicone. However, getting rid of all the residues can be a challenge. Be careful using silicone if a surface is to be coated or painted at a later stage. There are certain products that can help you by depolymerizing and dissolving silicone.
As the name suggests, hot melts can be melted again and again using heat (just make sure that you are not dealing with a curing hot melt adhesive!). Hence, if you’re having trouble removing the adhesive mechanically, heat is the key.
For more information about specific products and help choosing the right product, contact us and/or visit our main website.

Removing a cured adhesive can be tricky and the type will determine the method. The main methods are heat, chemicals and mechanical force.
Read more
When choosing an adhesive for your application, it is advantageous if you consider the adhesive viscosity to best suit your application and process.
Read more
Medical grade adhesives are commonly biocompatibility tested according to ISO 10993 standard. Choose Epoxy, UV-curing adhesive or silicone.
Read more
Electrically conductive adhesives are used in many different types of industries and applications. Some examples are electronics, solar cell, medical, aerospace, space and automotive applications.
Read more
Silicone rubber is one of the most difficult-to-bond rubbers. We will give you options for adhesives that can help you bond silicone.
Read more